KARALIV™
The Clinically Tested Herbal Formula
That Promotes Liver Function in 30 Days*
Command the Attention Your Brand Deserves!
More and more people are seeking dietary supplements to support healthy liver function. In fact, the global market for liver health supplements brought in an estimated $741.4 Million in 2020, and this market is expected to grow soon to $1 Billion.[1]
Nutraceuticals are in high demand for the liver health sector, so now is the time for formulators to look for innovative, efficacious and clinically studied ingredients for liver health. Command the attention your brand deserves and create a liver support product featuring KaraLiv™.
KaraLiv™ is a clinically tested and fast acting natural liver support formula that contains only pure and highly effective herbal extracts. Our product is available in powdered form for maximum potency and formulating flexibility, so it can be added to supplements, foods and beverages. Plus it's vegan, making it a clean and natural liver support option.*[2]
*These statement have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
This website summarizes the results of a randomized, double-blind, parallel and placebo-controlled study. KaraLiv™ is not offered for sale to consumers. The information contained herein is intended for use by manufacturers for product development purposes and should be reviewed by legal counsel before used on labels, in marketing or promotional materials or as support for any claim made in connection with a product that contains KaraLiv™.
Why Choose KaraLiv™
Clinically Tested Herbal Formula
Powdered Form for Formulating Flexibility
Safe & Highly Effective Vegan Formula
Non-GMO Project Verified, Kosher & HALAL
Fast Acting - Results in Less Than 30 Days*[2]
Promotes Liver Function*[2]
Maintains Healthy Liver Function*[2]
Supports Healthy Liver Function*[2]
The KaraLiv™ Clinical Study[2]
Many supplement manufacturers, distributors and consumers are often skeptical about the effectiveness of herbal ingredients for liver support, which is understandable. Very few, if any, herbal extract formulators can validate their marketing claims or the purity of their ingredients, but Karallief® IS NOT one of those companies. Karallief® believes in rigorous science and high quality ingredients.
The safety and efficacy of the KaraLiv™ formula was tested over the course of 56 days in a Randomized, Double-Blind, Parallel, Placebo-Controlled study on subjects with mild to moderately elevated liver enzymes.
One group of 30 study participants were given a 1,000 mg daily dose of KaraLiv™ (two 500 mg capsules/day) and the other group of 30 participants received a placebo. Primary treatment results were assessed in the study subjects in both groups by evaluating the levels of Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), S Alkaline Phosphatase (ALP) and Serum Bilirubin. Secondary results were assessed on Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Gamma-Glutamyl Transferase (GGT).
KaraLiv™ significantly outperformed the placebo group for both primary and secondary objectives[2]*
ALT: 40% reduction compared to 19% placebo – more than double the improvement*
AST: 53% reduction compared to 24% placebo – more than double the improvement*
ALP: 20% reduction compared to 10% placebo – double the improvement*
S bilirubin: 20% reduction compared to 12% placebo*
MDA: 27% reduction compared to 0.6% placebo – making KaraLiv™ 20x more powerful*
SOD: 20% increase compared to 0.9% placebo – making KaraLiv™ 20x more powerful*
GGT: 21% reduction compared to 4.5% placebo* – making KaraLiv™ 4x more powerful*
The KaraLiv™ Clinical Study Results[2]*
The KaraLiv™ group significantly outperformed the placebo group for the primary objectives, and below is an overview of the results.
40% Reduction in the KaraLiv™ Group vs 19% Placebo*
Alanine Aminotransferase (ALT)
In the KaraLiv™ group a reduction of ALT from a mean of 79.13 (baseline) to 61.63 (day 28) to 47.2 (day 56) was noted; a 40.35% reduction from baseline to the end of the study. The placebo group showed a reduction from a mean of 76.53 (baseline) to 65.96 (day 28) to 61.62 (day 56); a 19.48% reduction from baseline to the end of the study. Normal levels of ALT are below 25IU/L in females and below 33 IU/L in males.[3]*
53% Reduction in the KaraLiv™ Group vs 24% Placebo*
Aspartate Aminotransferase (AST)
In the KaraLiv™ group a reduction of AST from a mean of 122.4 (baseline) to 93.26 (day 28) to 57.16 (day 56) was observed; a 53.3% reduction from baseline to the end of the study. The placebo group had a reduction from a mean of 119.53 (baseline) to 102.31 (day 28) to 90.51 (day 56); a 24.28% reduction from baseline to the end of the study. Normal level of AST is under 40 IU/L in adults.[3]*
20% Reduction in the KaraLiv™ Group vs 10% Placebo*
S Alkaline Phosphatase (ALP)
In the KaraLiv™ group a reduction in ALP from a mean of 119.37 (baseline) to 105.3 (day 28) to 94.70 (day 56) was seen; a 20.67% reduction from baseline to the end of the study. In the placebo group, a reduction from a mean of 117.30 (baseline) to 109.27 (day 28) to 105.44 (day 56) was observed; a 10.11% reduction from baseline to the end of the study. Normal level of ALP is under 120IU/L.[3]*
20% Reduction in the KaraLiv™ Group vs 12% Placebo*
Serum Bilirubin
In the KaraLiv™ group a reduction in S Bilirubin from a mean of 1.2183 (baseline) to 1.0667 (day 28) to 0.97 (day 56) was observed; a 20.38% reduction from baseline to the end of the study.*
In the placebo group there was a reduction from a mean of 1.2033 (baseline) to 1.1140 (day 28) to 1.0524 (day 56); a 12.54% reduction from baseline to the end of the study. Normal range of serum bilirubin is 0.1-1.2 mg/dL.[3]*
The KaraLiv™ group also significantly outperformed the placebo group for the secondary objectives. Below is an overview of the results.
27% Reduction in the KaraLiv™ Group vs 0.6% Placebo*
Malondialdehyde (MDA)
In the KaraLiv™ group mean MDA level decreased from 3.44±0.43 to 2.51±0.37 from the baseline to the end of the study (a 27% statistically significant reduction).*
In the placebo group, mean MDA level decreased from 3.33±0.58 to 3.31±0.5 from baseline to the end of the study (not statistically significant).*
20% Increase in the KaraLiv™ Group vs 0.9% Placebo*
Superoxide Dismutase (SOD)
Mean SOD level in KaraLiv™ group showed a statistically significant increase from baseline (179.97±13.72) to the end of the study (216.13±20.84), a 20% increase.*
For the placebo the baseline and end of the study values were 205.76±26.23 and 207.52±27.87 respectively; but this change was not statistically significant.*
21% Reduction in the KaraLiv™ Group vs 4.5% Placebo*
Gamma-Glutamyl Transferase (GGT)
Mean GGT level in KaraLiv™ group at baseline and end of the study was 69.03±11.64 and 54.43±10.59 (a 21% decrease) while for the placebo group it was 66.52±11.64 and 63.48±13.32 respectively.*
Both groups showed a statistically significant decrease from baseline to the end of the study.*
The KaraLiv™ Clinical Study Showed
Our Liver Support Formula Is:[2]
Fast Acting – Experience results in less than 30 days!*
2x more powerful at reducing ALT, AST and ALP and 4x more powerful at reducing GGT compared to the placebo*
20x more powerful at reducing MDA and increasing SOD compared to the placebo*
A highly effective and safe option that’s natural and vegetarian/vegan*
KaraLiv™ Contains Clean Herbal Ingredients
While there are many manufacturers who use inferior herbal extracts and make unsupported claims about their products, we are not one of them.
Karallief® uses clean and potent herbal ingredients in our KaraLiv™ liver support formula. Our fast-acting proprietary blend has been scientifically studied and shown to help promote liver function in as little as 30 days!*[2]
Our herbal formula is truly unlike any other natural liver support product on the market. After extensive research and testing, our team of scientists developed a unique and synergistic formula based on specific ratios of standardized herbal ingredients that are known for supporting liver function.*
KaraLiv™ is in a powdered form as this provides maximum potency and shelf life. In addition, this delivery method lends the most flexibility to formulators, making it easy to add our product to anything from vitamins and supplements, protein powders, beverages and more. Plus, KaraLiv™ is vegetarian/vegan, making it a clean and natural liver support option.
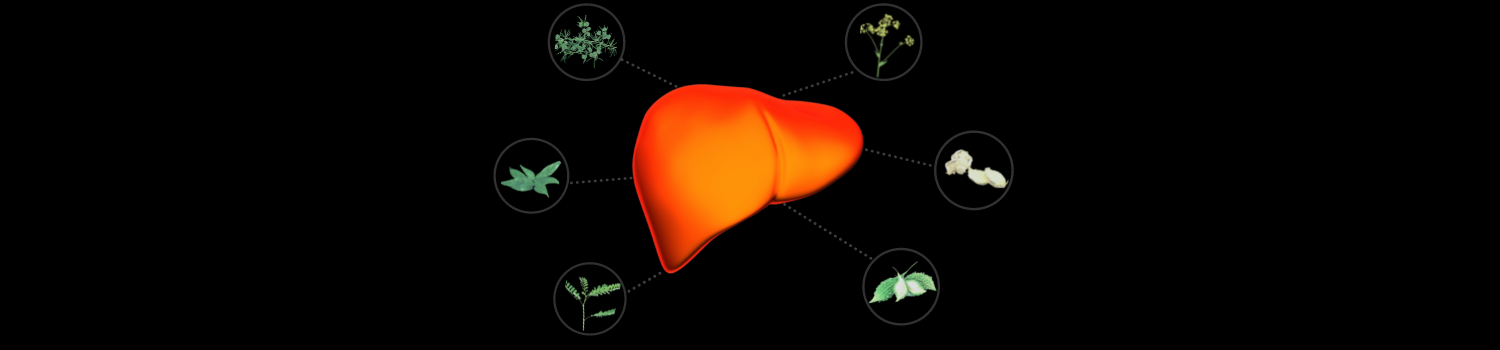
KaraLiv™ contains a potent 1,000 mg per day blend of the following:
Momordica Charantia
Helps Support the Liver and Normal Glucose Metabolism*[4-7]
Phyllanthus Niruri
Helps Support and Maintain Healthy Liver Enzyme Levels*[8,9]
Andrographis Paniculata
Helps Aid Digestion and Provides Liver Health Support*[10-13]
Brassica Rapa
Helps Provides Support for Healthy Liver Function*[14,15]
Asparagus Racemosus
Helps Support a Healthy Liver*[16]
Zingiber Officinale
Contains Agents that Provide Health Support for the Liver*[17,18]
Why Choose KaraLiv™
Clinically Tested Herbal Formula
Powdered Form for Formulating Flexibility
Safe & Highly Effective Vegan Formula
Non-GMO Project Verified, Kosher & HALAL
Fast Acting - Results in Less Than 30 Days*[2]
Promotes Liver Function*[2]
Maintains Healthy Liver Function*[2]
Supports Healthy Liver Function*[2]
Our Quality Standards and Protocols
Karallief® has successfully united the powerful forces of nature, science and innovation to develop our breakthrough KaraLiv™ formula.
Our company is unlike many ingredient suppliers that use inferior, untested materials that lack quality control measures. Instead, we pride ourselves on offering safe, efficacious and scientifically backed ingredients that are tested through thorough quality control procedures using high-end testing equipment.
Rigorous Quality Control
The testing performed covers a wide range of categories, including but not limited to: Physical parameters, chemical parameters, HPTLC chromatographic fingerprinting, HPLC quantified active compounds, impurity profile, microbial profile, residual solvents, residual pesticides, heavy metals profile and aflatoxins. The stability of every ingredient is periodically monitored through the product's lifetime and elaborate documentation is maintained to ensure traceability at all levels.
Comprehensive Quality Assurance
KaraLiv™ is manufactured in ISO 22000 and GMP certified facilities. There are specific HACCP and HARPC procedures that are followed to analyze each step in the production process. This enables us to assess the risk of any potential biological, chemical or physical hazard and contamination, and if one is identified, we implement specific control measures to address the issue, ensuring the product is manufactured safely, and free of hazards and cross-contamination.
Control Samples & Stability Testing
Control samples for every single batch of KaraLiv™ is maintained to facilitate traceability and to ensure that it consistently meets our high-quality standards. Stability testing of the product, which includes real-time and accelerated stability testing, is also performed at specified time intervals. By performing these tests, it helps us determine the shelf life of our products and monitor the characteristics of the product over time to ensure continued efficacy and purity.
Command the Attention Your Brand Deserves!
Create a Product with KaraLiv™

CONTACT US TO LEARN HOW WE CAN WORK TOGETHER
Karallief® Inc.
Email: KaraLiv@Karallief.com
Address: 75 Arlington Street, Suite 500, Boston, MA 02116, USA
Scientific References
https://www.researchandmarkets.com/reports/5302508/liver-health-supplements-global-market
https://www.ijbcp.com/index.php/ijbcp/article/view/4824
Healthline: Liver Function Tests. Available at https://www.healthline.com/health/liver-function-tests#types accessed on 16th June 2021.
Alam MA, Uddin R, Subhan N, Rahman MM, Jain P, Reza HM. Beneficial role of bitter melon supplementation in obesity and related complications in metabolic syndrome. J Lipids. 2015;2015:496169.
Zhang F, Lin L, Xie J. A mini-review of chemical and biological properties of polysaccharides from Momordica charantia. International journal of biological macromolecules. 2016 Nov 1;92:246-53.
Thomford KP, Thomford AK, Yorke J, Yeboah R, Appiah AA. Momordica charantia L. for hyperlipidaemia: A randomised controlled assessment of the Ghanaian herbal medicinal product MCP-1. Journal of Herbal Medicine. 2021 Aug 1;28:100453.
Saad DY, Soliman MM, Baiomy AA, Yassin MH, El-Sawy HB. Effects of Karela (Bitter Melon; Momordica charantia) on genes of lipids and carbohydrates metabolism in experimental hypercholesterolemia: biochemical, molecular and histopathological study. BMC complementary and alternative medicine. 2017 Dec;17(1):1-3.
Zarzour A, Hamdan R, Alshawsh MA, Asif M, Al-Mansoub MA, Mohamed Z, Ahmad M, Abdul Majid AM, Asmawi M, Kaur G, Al-Dualimi DW. Adipocytokine regulation and antiangiogenic activity underlie the molecular mechanisms of therapeutic effects of Phyllanthus niruri against non-alcoholic fatty liver disease. Nutrients. 2018 Aug;10(8):1057.
Ezzat MI, Okba MM, Ahmed SH, El-Banna HA, Prince A, Mohamed SO, Ezzat SM. In-depth hepatoprotective mechanistic study of Phyllanthus niruri: In vitro and in vivo studies and its chemical characterization. PloS one. 2020 Jan 15;15(1):e0226185.
Nyeem, M.A.B., Mannan, M.A., Nuruzzaman, M., Kamrujjaman, K.M. and Das, S.K., 2017. Indigenous king of bitter (Andrographis paniculata): A review. Journal of Medicinal Plants Studies, 5(2), pp.318-324.
Salunkhe AJ, Patil RN. Hepatoprotective effect of ethanolic extract of Andrographis paniculata against thioacetamide induced toxicity in the liver of albino rats. Research. Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences. 2018;4(6):549-59.
Liu YT, Chen HW, Lii CK, Jhuang JH, Huang CS, Li ML, Yao HT. A diterpenoid, 14-deoxy-11, 12-didehydroandrographolide, in andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrients. 2020 Feb;12(2):523.
Weng Z, Liu X, Hu J, Mu J, Xie J, Yao C, Li L. Protective effect of dehydroandrographolide on obstructive cholestasis in bile duct-ligated mice. Oncotarget. 2017 Oct 20;8(50):87903.
Naghdi N. Folklore medicinal plants used in liver disease: a review. International Journal of Green Pharmacy (IJGP). 2018 Nov 6;12(03).
Yin T, Bayanjargal S, Fang B, Inaba C, Mutoh M, Kawahara T, Tanaka S, Watanabe J. Lactobacillus plantarum Shinshu N-07 isolated from fermented Brassica rapa L. attenuates visceral fat accumulation induced by high-fat diet in mice. Beneficial Microbes. 2020 Nov 15;11(7):655-67.
Mishra JN, Verma NK. Asparagus racemosus: Chemical constituents and pharmacological activities—A review. Eur. J. Biomed. Pharm. Sci. 2017;4:207-13.
Dissanayake KG, Waliwita WA, Liyanage RP. A review on medicinal uses of Zingiber officinale (Ginger). Annu Res Rev Biol. 2020;10(6):142-8.
Morvaridzadeh M, Fazelian S, Agah S, Khazdouz M, Rahimlou M, Agh F, Potter E, Heshmati S, Heshmati J. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine. 2020 Nov 1;135:155224.
KARALLIEF® INC. 75 Arlington Street, Suite 500, Boston, MA 02116, USA | KaraLiv@Karallief.com
© Copyright 2024
Privacy Policy
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
This website summarizes the results of a randomized, double-blind, parallel and placebo-controlled study. KaraLiv™ is not offered for sale to consumers. The information contained herein is intended for use by manufacturers for product development purposes and should be reviewed by legal counsel before used on labels, in marketing or promotional materials or as support for any claim made in connection with a product that contains KaraLiv™.



![Momordica Charantia Helps Support the Liver and Normal Glucose Metabolism*[4-7]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1633538499455-68FOROQ9MEF0PJI7N2W7/Momordica+Charantia++%282%29.png)
![Phyllanthus Niruri Helps Support and Maintain Healthy Liver Enzyme Levels*[8,9]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1633538584238-6NOAA0WOHUORM27LWIN0/Phyllanthus+Niruri++%281%29.png)
![Andrographis Paniculata Helps Aid Digestion and Provides Liver Health Support*[10-13]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1633538634050-17HXLXUE80TEJSE2YHQI/Andrographis+Paniculata++%281%29.png)
![Brassica Rapa Helps Provides Support for Healthy Liver Function*[14,15]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1631568459698-CQAM9HNMZ6263SV0RWMB/Brassica+Rapa++%281%29.png)
![Asparagus Racemosus Provides Helpful Support to Protect the Liver from Damage*[16]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1631568626795-RSR0EXKDVU22W45D7Y42/Asparagus+Racemosus++%281%29.png)
![Zingiber Officinale Contains Agents that Provide Health Support for the Liver*[17,18]](https://images.squarespace-cdn.com/content/v1/6138ef5a7e183b7d779efb24/1633538846942-HYZNJ0GALXIEIIQCYQPS/Zingiber+Officinale++%283%29.png)



